May 29, 2024 - by Santina Russo
Palladium, rhodium, and platinum: As powerful chemical catalysts, these elements are key in many industrial processes. Chemical catalysts are most handy because they accelerate and drive chemical reactions without being consumed themselves. In fact, almost all processes in the chemical industry, as well as in the large-scale production of lab equipment, electronics, and many other products, rely on catalysts for efficient and steerable chemical reactions. However, many of these widely sought metals used as catalysts are amongst the rarest elements in the earth's crust, which in turn makes them some of the most expensive metals of all.
Moreover, their extraction is controversial since it is both expensive and polluting. The metals are typically found in geopolitically complicated locations where democracy is not in place or shaky and human rights are not always guaranteed. Consequently, the extraction of the metals has been harmful for workers involving a range of documented unfair labour practices up to child labour.
“The goal is therefore to find catalysts that are as effective as the ones used now, but without their problems,” says Michele Ceriotti, an associate professor at EPFL and head of the EPFL Laboratory of Computational Science and Modeling (COSMO). “More than that, we also want catalysts that reduce the energy consumption of chemical reactions.” To this end, in a project partly funded by PASC (Swiss Platform for Advanced Scientific Computing), and in collaboration with the chemical company BASF, Ceriotti’s team is investigating so-called high-entropy alloys.
High entropy: the more the merrier
“High-entropy alloys combine many different elements, typically five or more,” explains Ceriotti. These combinations come with decisive advantages: Firstly, such high-entropy alloys are stable and work well even in harsh reaction conditions, such as high temperatures, because high temperature generally stabilizes disordered systems; secondly, as several lab results have shown, they are very efficient and widely usable catalysts. Through the combination of many earth-abundant elements, the alloys can reach similar performances as today’s catalysts without using nearly as much rare materials — or even none at all. For instance, a high-entropy alloy might consist of 10 percent platinum that accumulates at the surface, and thereby prove as efficient as a catalyst consisting of pure platinum.
Unfortunately, the high entropy in their composition that makes these alloys ideal catalysts is also what renders them notoriously difficult to investigate. “The challenge is the immense number of possible combinations of elements which can in no way all be tested in the lab”, says Ceriotti. In addition, until now, no models were able to accurately describe and predict the behaviour of materials built of more than four of five elements in computer simulations.
Smartly reducing the complexity
To change this, Ceriotti and his team came up with a new concept — or rather an ancient one that they call alchemical compression: a sort of analogy to the concept of the basic alchemic elements earth, water, air, and fire. “Early naturalists and scientists used these classical elements to explain the complexity of matter in terms of a few simple substances,” says Guillaume Fraux, a research software engineer in EPFL’s high-performance computing team. “Everything in nature was thought to be composed of different portions of these four classical elements.” In a very similar way, the teams’ novel model treats each chemical element as a combination of three or four simplified “pseudo elements”.
“This reduces the overall complexity of the problem drastically since the individual chemical elements are not looked at as independent entities, but rather as correlated by their common make up from the pseudo elements — like a kind of common ancestry”, explains Fraux. This method simplifies handling the interactions between the elements. Consequently, computational cost is reduced, which makes modelling of such high-entropy alloys finally feasible. And, even with the simplification, results are remarkably accurate.
In practice, the team used CSCS’s supercomputer “Piz Daint” to train their ML model with approximately 25,000 structures composed of three to 25 randomly picked elements from the d-block of the periodic table, known as the transition metals. The ML model went through the structures and learned correlations using the concept of the pseudo elements. Over several iterations, the model learned to reproduce the pre-calculated energy of each structure using this simplified pseudo element representation.
From validation to prediction
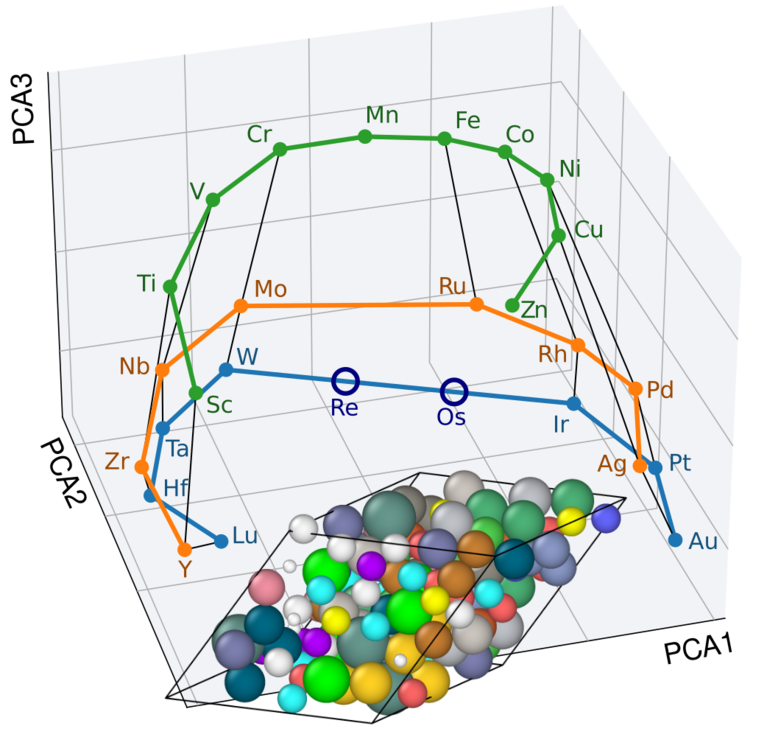
Once trained, the model independently rediscovered the similarities between different elements and grouped them in rows and columns, effectively reproducing, in large parts in fact, the periodic table. Moreover, as validated by the scientists, the ML model can predict properties of compounds it was not trained with, based on the similarities with those included, namely the correlations between the elements based on the pseudo element principle.
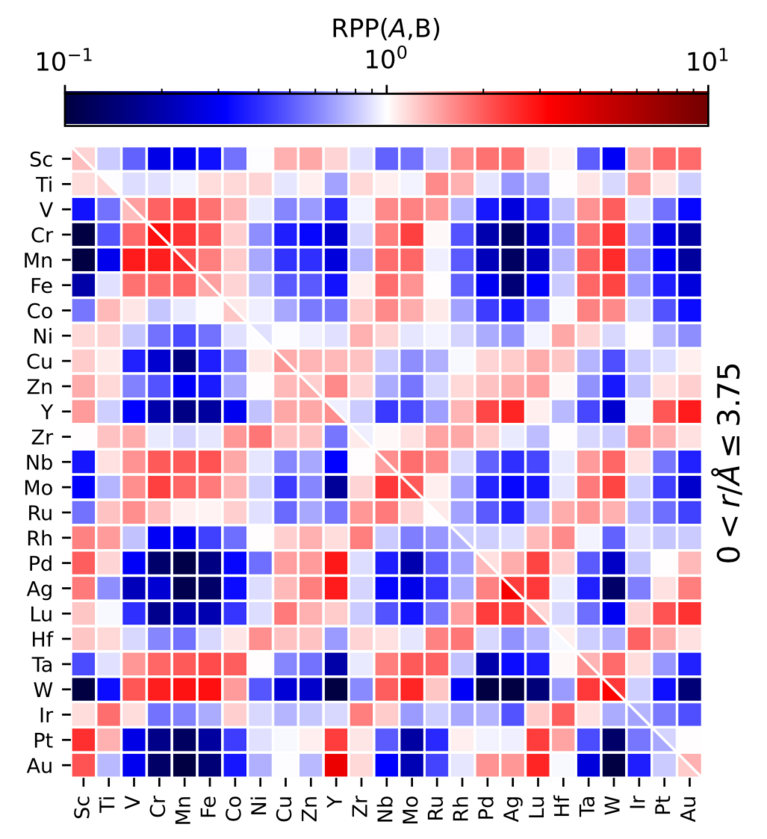
After validating the prediction power of their ML model, the team went on to use it in computational experiments, also performed on CSCS’s “Piz Daint”. One of the fundamental results of these simulations was the observed tendency of the elements’ distribution in high-entropy alloys. “Contrary to what was long assumed, the different atoms are not randomly distributed in the materials,” says group leader Ceriotti. “In fact, there are strong preferences.” If one looks at the element manganese (Mn), for instance: It is far more likely to be located near vanadium (V), chromium (Cr), or Iron (Fe) than it would in a random distribution. In contrast, Mn it is far less likely than random to find itself near rhodium (Rh), palladium (Pd), silver (Ag), or lutetium (Lu).
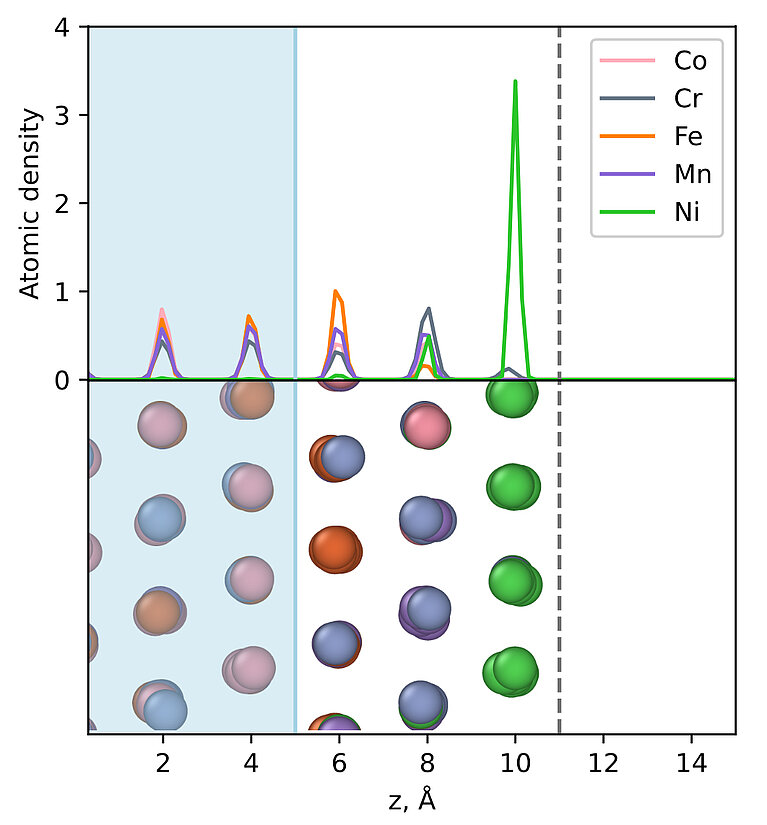
The team went on to analyse specific alloys, such as one composed of cobalt (Co), chromium (Cr), iron (Fe), manganese (Mn) and nickel (Ni), and to explicitly determine the propensity of the various components to accumulate at the surface. As the simulations showed, this CoCrFeMnNi alloy’s different elements are not at all randomly distributed. Most notably, the nickel atoms all accumulate at the surface of the material. Meaning that, at the surface, where in a chemical reaction this catalyst would be in contact with other compounds, the properties of nickel would be dominant.
Towards a more sustainable industrial chemistry
“This prediction power can now be used to design the catalysts we wish for,” says Ceriotti. “Different compositions can be tested to find those in which a specific desired element is predominantly located at the surface — and in a much more efficient as well as more accurate way than with classical simulations, and almost as accurate and faster than with quantum simulations.”
The goal: To replace as much of the powerful, but ecologically and socially problematic elements like palladium, rhodium, or platinum as possible. To this end, in the application side of the project, Ceriotti’s team is collaborating with the chemical company BASF. “Chemical companies have a big interest in catalysts that are more efficient and less energy consuming than the ones available to date,” Ceriotti explains. “Since they are producing in high volumes, even a one-percent savings has an immense impact on their production costs.”
The newly developed ML model and method based on correlations via pseudo elements is already speeding up these kinds of calculations immensely, making them around 100,000 times faster. Furthermore, in a project supported by the PASC program, Ceriotti’s team is working on optimizing their code to use GPUs as efficiently as possible, especially the GPUs and parallelization capabilities of CSCS’s brand new research computing infrastructure, Alps.
(Image above: The image shows a palladium mineral rock. Palladium is one of the most precious materials in the world, costing more than gold or platinum. The noble metal is widely used as a catalyst. It is largely extracted as a by-product of nickel and copper mining, particularly in mines in Siberia and South Africa).
References:
- N. Lopanitsyna, G. Fraux, M.A. Springer, S. De, and M. Ceriotti: Modeling high-entropy transition metal alloys with alchemical compression, Phys. Rev. Materials (2023), DOI: https://doi.org/10.1103/PhysRevMaterials.7.045802
- A. Mazitov, M.A. Springer, N. Lopanitsyna, G. Fraux, S. De, and M. Ceriotti: Surface segregation in high-entropy alloys from alchemical machine learning, J. Phys: Mater (2024), DOI: https://doi.org/10.1088/2515-7639/ad2983